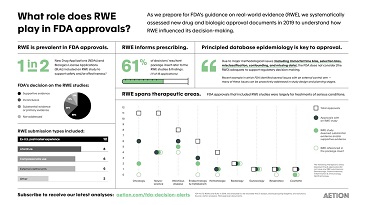
RWE is now helping the U.S. Food and Drug Administration (FDA) to approve more drugs than ever. In 2019, RWE studies were included in 50% of the agency’s approvals. Aetion is currently evaluating approval documents for New Drug Applications (NDAs) and Biologics License Applications (BLAs) to better understand the role of RWE in the agency’s decision-making. This infographic provides an overview of Aetion’s research including how prescribing is informed by RWE, the therapeutic areas and submission types that most often use RWE, and the main considerations for generating decision-grade evidence. Read more and download the infographic here.
RWE is now helping the U.S. Food and Drug Administration (FDA) to approve more drugs than ever. In 2019, RWE studies were included in 50% of the agency’s approvals. Aetion is currently evaluating approval documents for New Drug Applications (NDAs) and Biologics License Applications (BLAs) to better understand the role of RWE in the agency’s decision-making. This infographic provides an overview of Aetion’s research including how prescribing is informed by RWE, the therapeutic areas and submission types that most often use RWE, and the main considerations for generating decision-grade evidence. Read more and download the infographic here.
(Source: Christina Purpura, Aetion, September 24, 2020)