Big Ideas for Big Data Analytics in Real-World Evidence: Insights from Dr. Rich Gliklich, CEO of OM1
August 9, 2017

HealthEco
[HE.Com] With the influx of big data, how do you think the pharmaceutical industry’s approach to real-world evidence should evolve? Are patient registries still relevant?
[RG] Healthcare data is growing at an astounding rate. This creates both challenges and opportunities. Pharma is being asked to demonstrate value to more and more stakeholders. We define Value as: Value = Outcomes/Cost, and thus, Pharma will increasingly seek deeper clinical information and true outcomes to help demonstrate value and justify their prices.To get to this critical evidence, they will need to turn to highly specialized capabilities and data for particular conditions, and they will need these data regularly updated, because the dynamics in the market change. For most organizations, it will be more cost-effective and timely to subscribe to sources that track and analyze these changes, than to build out the infrastructrure and acquire the data independently, such as from clinical trials and prospective patient registries.
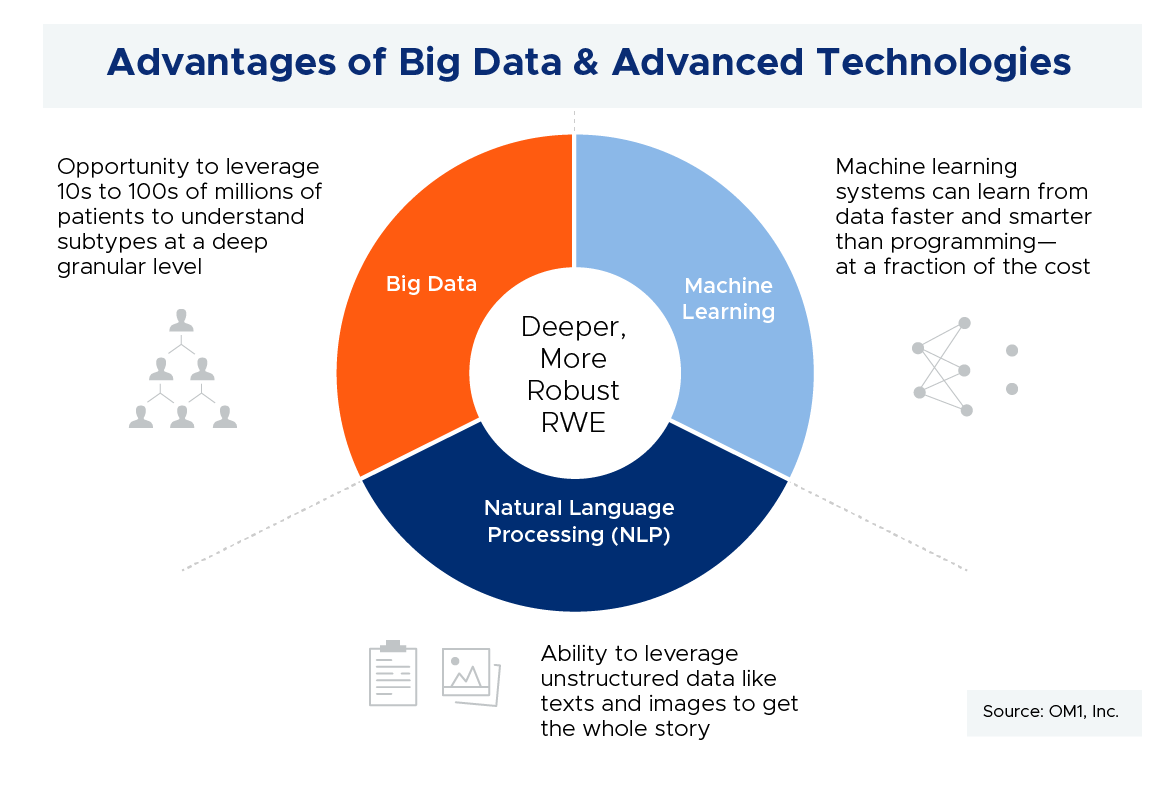
The goals of patient registries are still relevant—from understanding the natural history of a disease to meeting a post market safety commitment. But, big data plus advanced technologies creates alternative opportunities to meet those goals across many therapeutic categories and conditions, and to do so with very large patient numbers (orders of magnitude more patients vs registries), and at a fraction of the cost.
[HE.Com] Are there disease or therapeutic areas where using big data, as you’ve described, may be more effective? What types/level of disease and patient level data could we get to that is different than what we have access to today with traditional registries and trials?
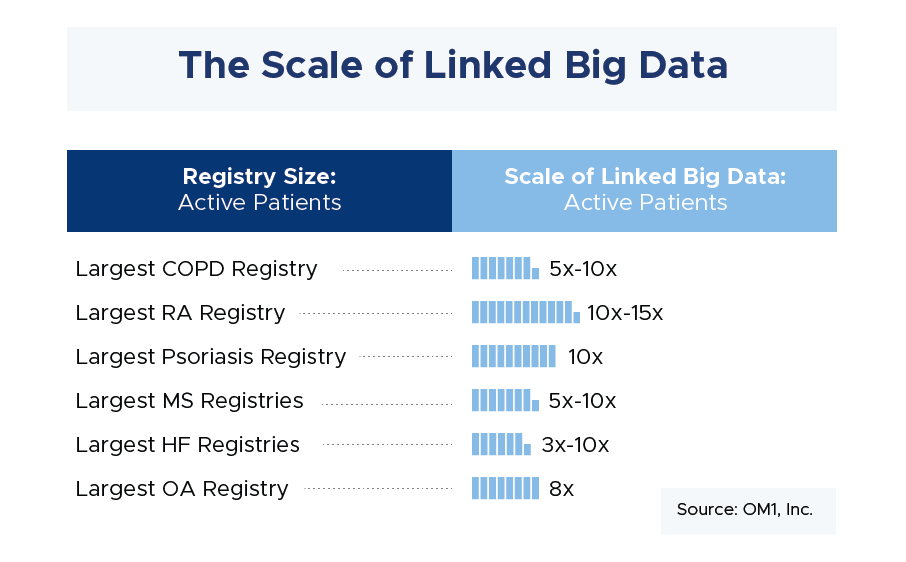
[RG] Conditions where comprehensive patient journeys can be readily captured through linking different data sources will be most amenable to using big data. There are a number of conditions where this is the case, from airway to cardiovascular and immunologic diseases to name a few. And, the number of such conditions with comprehensive patient journeys using disparate data linking is growing every day. Beyond traditional clinical and laboratory data available through registries and trials, we are now able to collect more and more patient-generated data, cost information, socioeconomic data, mortality, and so on. In addition to a more thorough understanding of the patient experience, these approaches also have the advantage of collecting information on all patients. This removes some of the enrollment biases we see in both registries and trials. The graphic below demonstrates the large sample size that can be attained through linked data compared with the sample size from a typical prospective registry.
[HE.Com] Recently STAT News reported that Actemra® (tocilizumab) was responsible for hundreds of deaths and that the risks for life threatening complications were as high or higher than competing products. Could you comment on this finding, and is there a better way to look at this situation, using a big data perspective?
The STAT report was based on the FDA Adverse Event Reporting System. The problem with those systems is that they are based on voluntary reporting and there is no denominator to actually determine the incidence of the events. This creates potential bias in reporting, since clinicians who are aware of a potential problem with a drug are then more likely to report events associated with it. Using big data, we can have a true denominator where the number of exposed patients and the number of events are both known, and these are derived from a combination of data sources.
In response to the STAT report, OM1 rapidly analyzed 120,000 patients on DMARDS (disease-modifying antirheumatic drugs) over the past 12 months to assess these findings in a more systematic and controlled analysis using big data. We did not find the same difference in event rates when patients carefully adjusted for comorbidities. Since the results are not yet published, I cannot go into details, but it demonstrates the power of using big data to respond rapidly to safety and other signals that may come up from time to time.
[HE.Com] How will payer needs for RWE evolve over the next 5 years, and what are the opportunities for service providers (pharma, researchers, data analytics companies) to address these future needs?
As payers move towards value-based care, they need a deeper understanding of clinical outcomes beyond what is available in their own claims data sets. While the goal is to understand the clinical outcome, meaning the patient and provider-relevant impact of the treatment on the patient, the actual results today are tabulated from what transactions were billed for by the doctor or hospital. As a result, these outcomes have generally focused on easily measured billing items such as hospitalizations, adverse event rates or complications of procedures.
But data derived from these sources lacks clinical depth and nuance. Critical questions are unanswered when using billing data for outcomes assessment. How serious was the hospitalization? Did the patient return to normal functioning and well-being? Did the underlying disease process improve or worsen as result of the treatment? None of this is actually measurable in claims data. These shortcomings are leading payers and pharma to seek more clinically-focused RWE, and each will do it for their own purposes. Payers will also increasingly partner with pharma to support value-based assessments and outcomes based contracts.
[HE.Com] How will patients’ use of big data evolve? Are there case studies of patient use that will become the norm for other disease states in the future?
Both patients, and providers on behalf of patients, will use big data to personalize healthcare. Big data analytics that are focused on outcomes will support better decision-making for patients. As a result, patients will have the opportunity to better understand their own specific risks and benefits with respect to different therapies, as well as their likelihood to achieve both positive and negative outcomes. Let me give you a generalized example. In the future, a patient with rheumatoid arthritis will be able to know that for patients like them, 80% improved with drug A vs. 40% with drug B and these results were derived from broad big data rather than limited clinical trials. In this scenario, a typical patient will have much more information to make informed choices about their own or their loved one’s healthcare.
[HE.Com] What regulatory hurdles or opportunities do you see as they relate to big data, RWE, or related evidence dissemination?
Regulatory interest in big data and RWE is at an all time high as recent regulations such as the 21st Century Cures Act are driving regulators to consider RWE as a potential replacement for other forms of evidence generation around and post-approval. In Section 505F of the Cures Act, it states, “The Secretary shall establish a program to evaluate the potential use of real world evidence (1) to help support approval of a new indication under section 505(e); and, (2) to help support or satisfy post-approval study requirements.” The program is to be implemented “within 2 years” and ‘real world evidence’ is defined as data “from sources other than clinical trials”. At the same time, some of these new paths to use RWE for these purposes are untested and there will certainly be some growing pains. Examples of such hurdles are seen in the work we do with outcomes-based contracting where issues regarding measurement outside of the label and Medicaid best price continue to create barriers.
As researchers, we are doing our part to facilitate better informed decisions for individual impact, using our intelligent data cloud to transform population data into precision health. And we’re trying to get deeper into clinical and patient data, more quickly. But there is much work to be done.
[HE.Com] Thank you, Rich. For more information on the work of Dr. Gliklich and colleagues in the area of RWE, view the recent on-demand webinars by clicking on the links below.
Rich is the CEO of OM1, a healthcare technology company focused on understanding the patient journey and the true results of healthcare. Since 2014, Rich has been an XIR at General Catalyst, where he supports businesses in the healthcare industry. Prior to joining General Catalyst, Rich was President of the Outcome division of Quintiles, the largest provider of biopharmaceutical development and commercial outsourcing services, and he also served on its Executive Committee through its 2013 IPO. Prior to Quintiles, Rich was Founder, CEO and Chairman of Outcome, a health information and services company that served more than 2,500 healthcare organizations and a majority of the global top 30 life sciences companies. Rich led Outcome from its start as a spin-off from his Harvard affiliated research laboratory in 1998 through its acquisition by Quintiles in October 2011. In addition to his experience as an entrepreneur and executive, Dr. Gliklich is well known in the areas of registries, outcomes and analytics. He is senior editor of the landmark publication by the U.S. Agency for Healthcare Research and Quality (AHRQ) handbook “Registries for Evaluating Patient Outcomes: A User’s Guide” and the PI for the Outcomes Measures Framework, which focuses on standardization of outcomes measurement. Rich has led several key national and international efforts focused on evaluating the safety, effectiveness, value and quality of healthcare. Rich also holds several patents for both health outcomes systems and medical devices. Rich is a graduate of Yale University and Harvard Medical School and a former Charles A. Dana Scholar at the University of Pennsylvania. Rich is also a surgeon and the Leffenfeld Professor at Harvard Medical School.
If you or someone you know would like to be featured in the HealthEconomics.Com CEO Profiles Series, contact Dr. Patti Peeples, CEO of HealthEconomics.Com.